Apple Watch ECG will soon work at higher heart rates of up to 150BPM
The Apple Watch will soon be able to detect atrial fibrillation via the ECG function at higher heart rates than at present, potentially allowing for someone who was working out to use the app without relaxing beforehand.
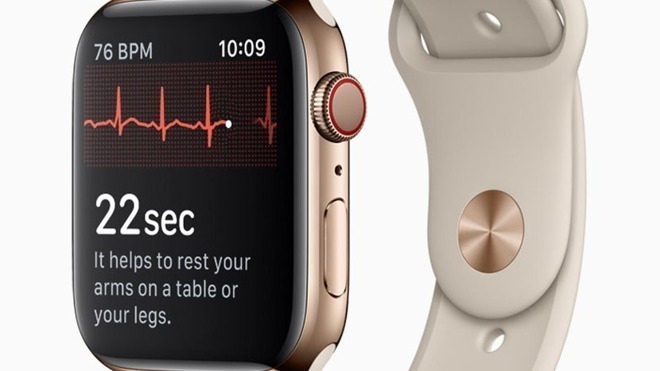
Introduced with the Apple Watch Series 4, the ECG app can record the electrical signals generated by a person's body during a heartbeat, using two points of contact. While the app has been repeatedly credited with helping to detect heart problems, like AFib, the current version of the app relies on the user having a more manageable pulse in order to perform the reading.
The existing version offers an "inconclusive" reading for attempts to record a heart rate below 50BPM or over 120BPM, which means it can't be as effectively used by those on certain medications that slow the heart, or are an elite athlete who can naturally acquire a low heart rate through training. It also means that those who have an elevated heart rate following exercise or gain it through various factors, such as stress, nervousness, or alcohol, cannot use the feature.
According to MyHealthApple, Apple applied to the FDA for an update to its ECG app in August, and was granted approval for its changes. These alterations, which may appear in the watchOS 7.2 update anticipated to ship in the coming week, includes expanding the range it can perform an ECG reading to a maximum of 150BPM.
For those who do have an AFib reading with a heart rate between 100 and 150bpm, there will be a new classification that denotes it as a reading with the high rate.
Screenshots from watchOS 7.2 were posted to Twitter by Steve Moser, who warns the feature may not be available in all locations. Given the FDA approval, as well as a change in algorithm spotted on Wednesday, it seems highly likely it will be available to US-based users, though further approvals may be required in other territories, such as Taiwan.

Introduced with the Apple Watch Series 4, the ECG app can record the electrical signals generated by a person's body during a heartbeat, using two points of contact. While the app has been repeatedly credited with helping to detect heart problems, like AFib, the current version of the app relies on the user having a more manageable pulse in order to perform the reading.
The existing version offers an "inconclusive" reading for attempts to record a heart rate below 50BPM or over 120BPM, which means it can't be as effectively used by those on certain medications that slow the heart, or are an elite athlete who can naturally acquire a low heart rate through training. It also means that those who have an elevated heart rate following exercise or gain it through various factors, such as stress, nervousness, or alcohol, cannot use the feature.
According to MyHealthApple, Apple applied to the FDA for an update to its ECG app in August, and was granted approval for its changes. These alterations, which may appear in the watchOS 7.2 update anticipated to ship in the coming week, includes expanding the range it can perform an ECG reading to a maximum of 150BPM.
For those who do have an AFib reading with a heart rate between 100 and 150bpm, there will be a new classification that denotes it as a reading with the high rate.
Screenshots from watchOS 7.2 were posted to Twitter by Steve Moser, who warns the feature may not be available in all locations. Given the FDA approval, as well as a change in algorithm spotted on Wednesday, it seems highly likely it will be available to US-based users, though further approvals may be required in other territories, such as Taiwan.

Comments
Regulatory approval is conditioned on specific parameters in which supporting evidence has been provided that shows within a certain parameter, the diagnosis is accurate. It could be that no evidence was submitted for the regulatory approval for the ECG ability above or below a certain BPM and therefore FDA approval for the ECG in the Apple Watch was only for between specific BPM parameters.
(Actually, I also don't know if this is an FDA approval or an FDA clearance; they mean different things.)
Second, @svanstrom is right - if you really are worried about your rhythm and it's 180 you should be getting evaluated by something other than your watch. Also, EKGs become difficult to read if the heart rate is above 150~160, so I would trust a watch even less for those.
Having said that, bradycardia rhythms should be manageable by Apple watch and seeing the difference between sinus bradycardia (for example from good conditioning) and something more pathologic would be beneficial. From the sounds of the article, Apple only got FDA approval for certain heart ranges initially and they are now simply trying to expand that approval..